Bottling represents the last stage of the oenological process, and its success depends to a great extent on the precise management of dissolved gases, mainly oxygen (O₂) and carbon dioxide (CO₂). Their correct balance not only guarantees the aromatic and visual stability of the wine, but also its conservation, typicity and bottle aging capacity.
While the rest of the winemaking process is carried out under the direct control of the winemaker, bottling involves momentary exposure to higher risk conditions: oxygen ingress, loss or excess of CO₂, and variations related to the type of closure. Therefore, its planning must be contemplated with the same rigor as any other critical winemaking phase.
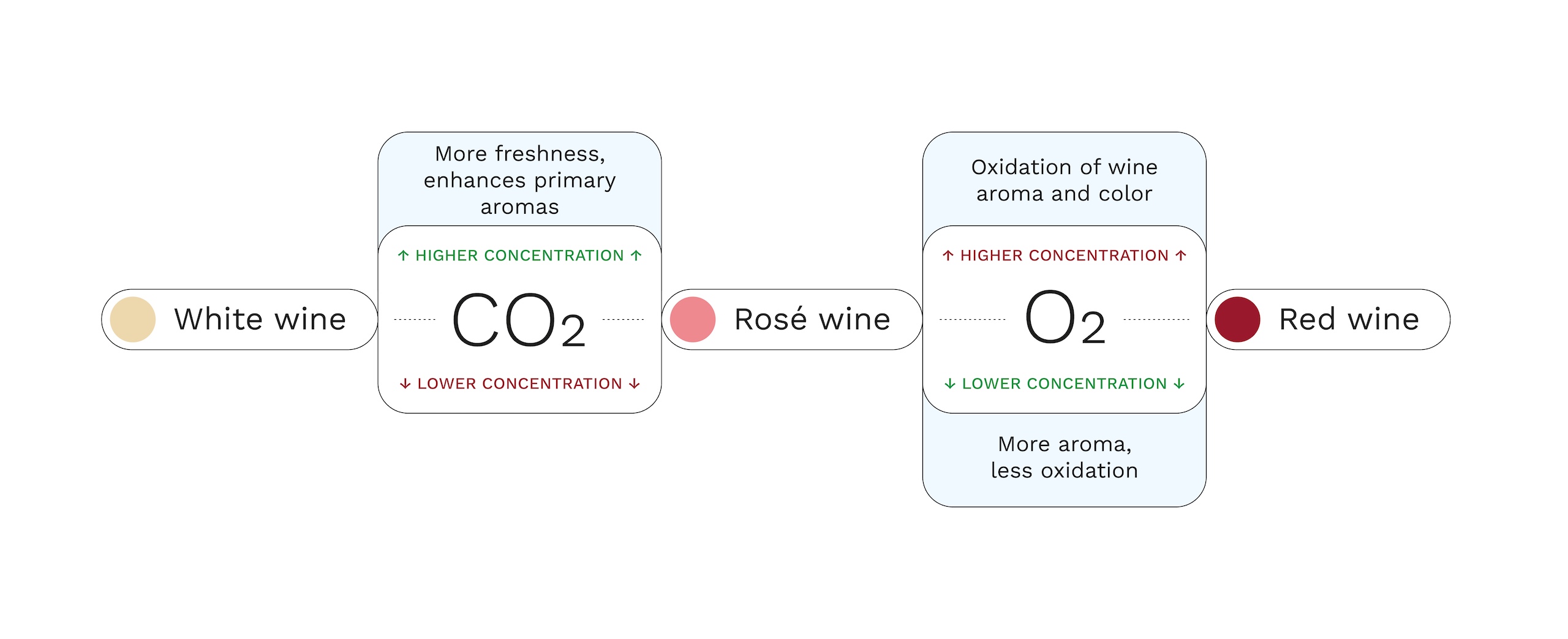
The relevance of carbon dioxide
CO₂ directly influences the perception of wine. In reds it can accentuate the sensation of astringency and bitterness if it is not controlled; on the other hand, in whites and rosés it endows freshness and vivacity. Therefore, it is recommended to adjust CO₂ levels according to the style:
- Reds: 0.2-0.4 g/L to avoid aggressive sensations.
- Young whites/rosés: 1.5-2 g/L to maintain freshness.
Influence of oxygen during winemaking
O2is an indispensable molecule in winemaking: it contributes to color stabilization (polyphenol polymerization reactions) and favors the achievement of higher electrochemical potentials that prevent the formation of odorant compounds of reductive origin, polymerization of “hard” or “astringent” tannins to “sweeten” them, among other aspects. In alcoholic fermentation, it helps the synthesis of fatty acids and cell membrane sterols, facilitating yeast activity.
However, dissolved oxygen is responsible for most oxidation phenomena, which include aromatic losses and evolution as well as browning and color losses. These phenomena are most relevant at the time of bottling.
A certain amount of oxygen is added at each stage of the winemaking process, and saturation levels can be reached. The following table shows some values of these contributions:
|
Operation
|
O₂ Input [mg/l]
|
Source
|
|---|---|---|
|
Racking
|
3 – 4
|
E. Peynaud
|
|
Racking
|
2 – 6
|
Vivas (1997)
|
|
Homogenization
|
2 – 4
|
Agrovin (2009)
|
|
Pumping (depending on the pump)
|
0.2 – 3*
|
INRA (2001)
|
|
Microfiltration
|
0.2 – 4*
|
INRA (2001)
|
|
Cross-flow filtration
|
1.5
|
Vidal et al. (2001–2004)
|
|
Centrifugation
|
1.2
|
Castellari et al. (2004)
|
|
Continuous tartaric stabilization
|
4.0
|
Castellari et al. (2004)
|
|
Tartaric stabilization by cold treatment
|
2.38
|
Vidal et al. (2001–2004)
|
|
Barrel blending
|
1.75
|
Castellari et al. (2004)
|
|
Bottle filling
|
0.3 – 1.3
|
INRA (2001)
|
Table 1: Oxygen contribution in the different cellar operations.
The role of oxygen during bottling
In the case of bottling, the aim is to leave sufficient levels of free SO2for the wine to be preserved over time. This is a delicate task, as low levels will not protect the wine for the necessary time and high levels can lead to unpleasant odors.
There are three sources of oxygen in the bottle:
- Headspace, which cannot be controlled beyond the bottler’s design. This oxygen is consumed in a month and a half, the amounts can vary from 0.6 to 3 mg/l (Vidal J.C. et al. 2004).
- Dissolved oxygen (DO) in the bottling process is consumed in about 2 weeks. 0.9-6 mg/l (Vidal J.C. et al. 2004).
- Oxygen entering through the closure which will consume all the free SO2in the wine. Depending on the type of closure, this process has a variable duration, from months to years. This process is also unavoidable. DO input through the closure: 0.2-15 µl/day in non-defective closures, while in screwcaps the transfer is much lower.
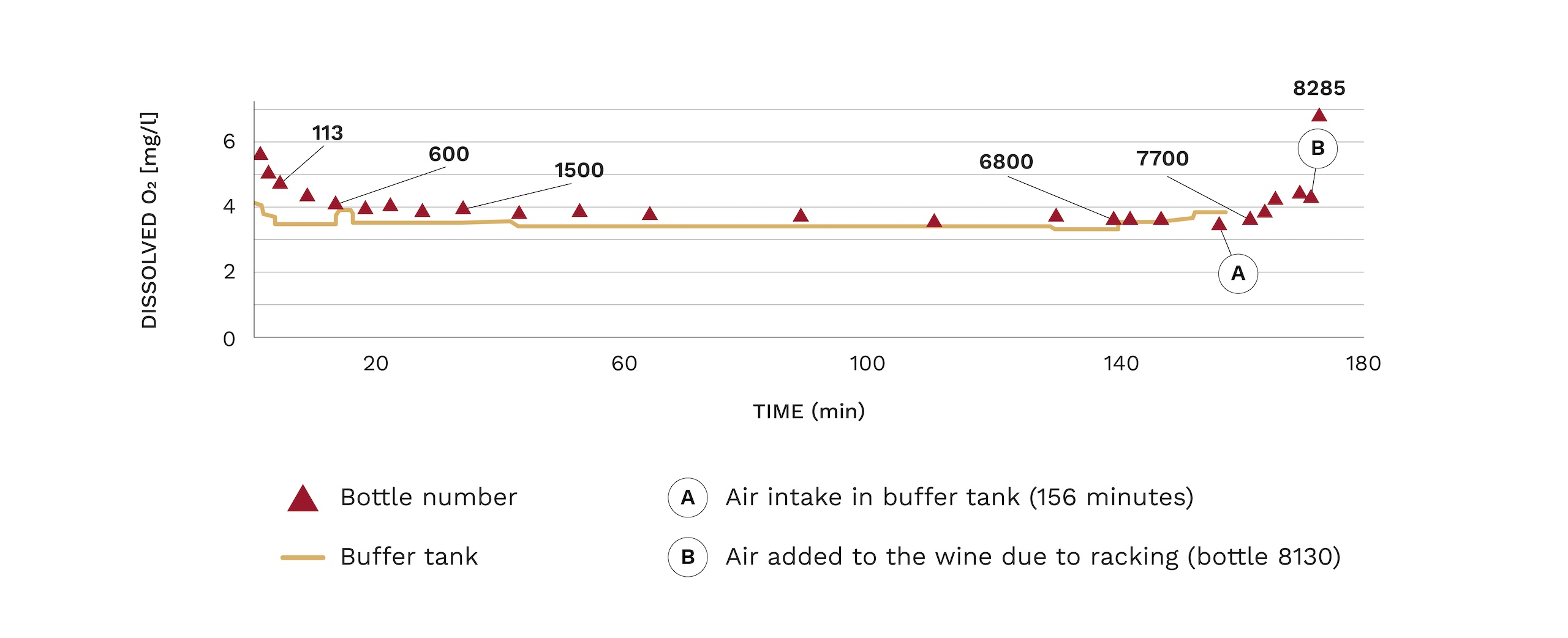
The DO varies during the bottling process; at the beginning it increases due to the filling of the line and filters and the formation of air pockets in the piping. At the end of the process an increase in DO is also observed due to the tightening of the tank and subsequent pushing. This increase can be seen in the following figure:
Figure 1: Evolution of dissolved oxygen content during bottling
Source: Vidal, J.C., Boulet, J.C., Moutonet, M., INRA (2004)
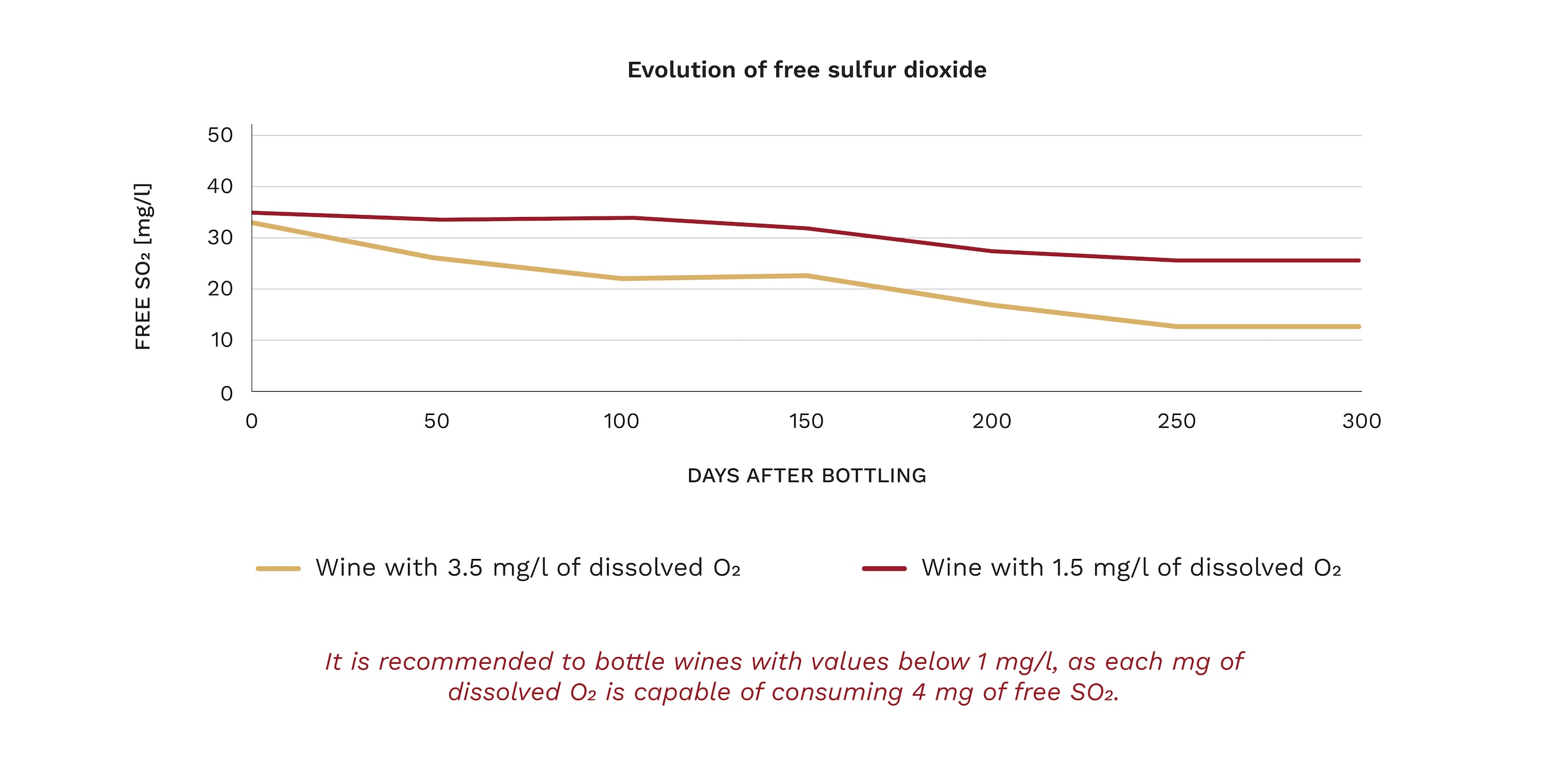
In white wines, it has been shown that the oxidized character in wines appears at free SO2levels below 10 mg/l. In the case of red wines, this behavior is somewhat different since polyphenols have antioxidant activity.
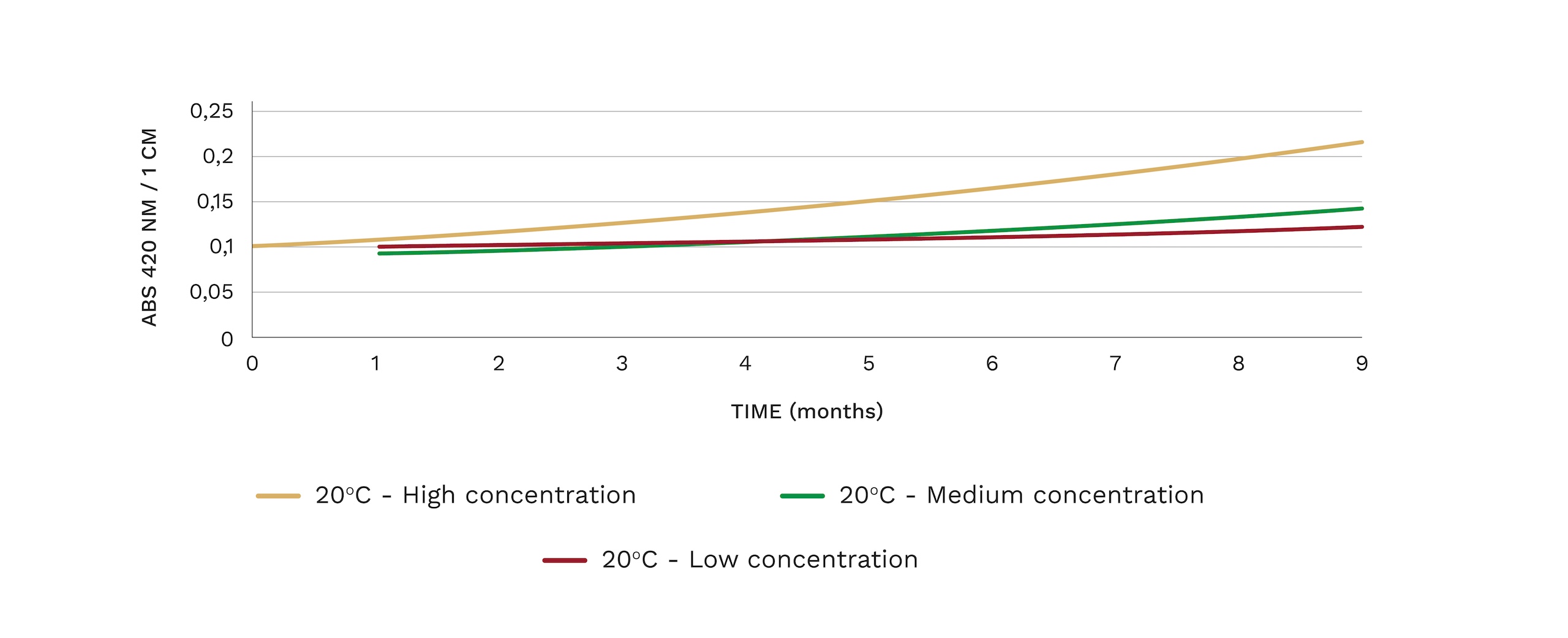
Figure 2: Evolution of the yellow component (Abs 420nm) of a white wine with different DO levels at the time of bottling; Low concentration < 1 mg/l ; Medium concentration = 3 mg/l; High concentration > 5 mg/l.
Source: Vidal, J.C.; Boulet, J.C.; Deage, M.; INRA (2004).
Each mg of dissolved O2is capable of consuming 4 mg of free SO2, so it is essential to eliminate it before bottling so that the wine can evolve more slowly, respect the aromas and color, especially in white and rosé wines, so that the winery can bottle with lower levels of free sulfur.
How can membrane contactors help us to control oenological gases dissolved in wine?
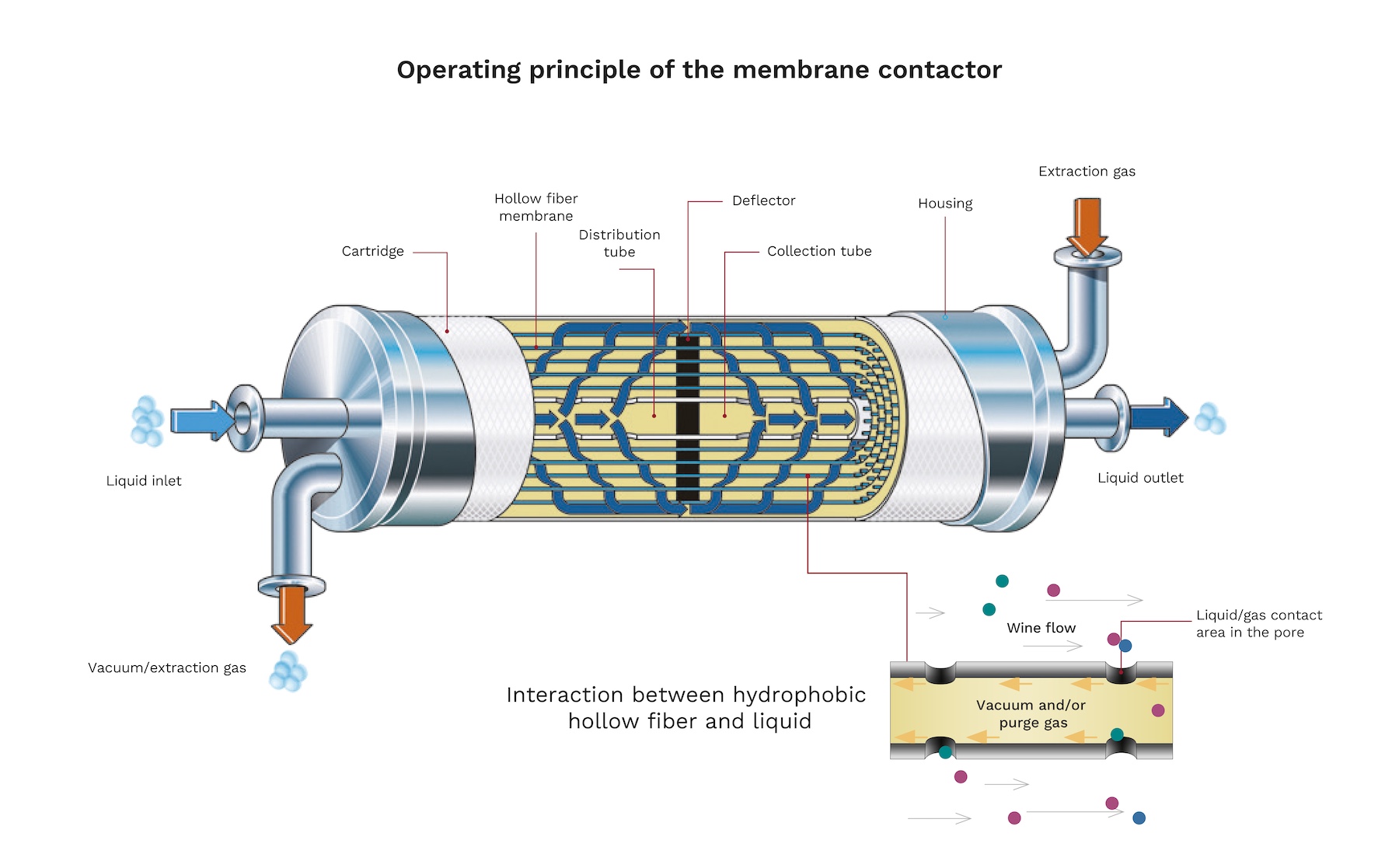
There are numerous methodologies for the control of dissolved gases in wine prior to bottling, however, the development of membrane contactor technology has made them a highly effective tool at the industrial level.
Contactors are cylindrical structures made up of thousands of hollow micro-perforated food-grade polypropylene fibers with a high hydrophobic character. Each fiber has an inner diameter of 200 µm and an outer diameter of 300 µm, with a pore diameter of 0.03 µm. This small pore size allows only low molecular weight gases (O2, CO2 and N2) to pass through.
The principle of operation of the contactors is simple. Due to the hydrophobic character of the membrane, at no time does the wine come into contact with the inert gas, the membrane acts as a support between the liquid-gas phase. By adjusting the partial pressure of the gas, the gases dissolved in the wine can be selectively removed or dissolved, this process will be governed by Henry’s Law.
The variables that will influence the performance of the process will be the following:
- Wine flow rate.
- Inert gas flow rate.
- Initial concentration of the gases.
- Temperature.
- Gas pressure.
Membrane contactors are useful for gas management at bottling and other processing stages, and are particularly suitable for these applications.
O2 removal
Bottling with high dissolved O2 values will lead to color and aroma development problems. Browning and oxidation aromas will appear quickly, as SO2 will combine rapidly and its protective capacity will disappear.
Therefore, the use of membrane contactors during winemaking will help us to reduce the amount of dissolved oxygen, keeping the wines protected even with lower SO2 values, and their use is particularly interesting at the following moments:
Bottling: decrease of up to 80% of dissolved O2 present in the wine.
- Loading-unloading of tanks.
- Wine inerting: saturation with 50% less N2 consumption than in the case of using bubblers.
- Racking of barrels: reduction of dissolved O2, which can reach up to 6 mg/l.
- Cold stabilization: reduction of dissolved O2 that can reach saturation values (11.2 mg/l at 0ºC).
CO2 removal and supply
The management of carbon dioxide in wine is complicated by its high solubility and high temperature dependence. However, the use of membrane contactors makes it possible to work with great precision and efficiency to adjust CO2 levels at bottling to the most advisable levels.
- Bottling: decrease of dissolved CO2 by up to 40%.
- Bottling: increase of CO2 up to 2.4 g/l at atmospheric pressure.
Oxi Out uses membrane contactor technology for precise management of dissolved gases. The components that form it allow us to know accurately and on-line the concentration of O2 and CO2, as well as the temperature at which we are performing the process and the pressure of the system.